Starch 1500®
Partially Pregelatinized Maize Starch
Starch 1500® enhances stability of moisture-sensitive drugs and ensures excellent content uniformity. It lowers costs by reducing or eliminating binders, superdisintegrants and excessive levels of lubricants and glidants.
- Starch 1500 is US-made product. In the condition of not violating any applicable sanctions or regulations, we can provide services for technical inquires, quality or other relevant demands for returning customers.
- Please don't hesitate to contact us if you need any help.

Uniquely Colorcon
Starch 1500® is a partially pregelatinized maize starchmanufactured exclusively for the pharmaceutical industry indedicated GMP facilities.
Through a unique manufacturing process, the bond betweenthe two polymers of maize starch,amylose and amylopectinis broken. This provides Starch 1500 with unique properties:good compactability, flow and lubrication.
With over 50 years history, Starch 1500 has been used inthousands of marketed products in innovator, generic, OTCand nutritional market segments across the world.
Starch 1500 continues to mitigate some of the mostsignificant risks formulators and manufacturers are facing.
Excellent Stability for Moisture Sensitive Drugs
Starch 1500 enhances the stability of moisture sensitive
drugs by inhibiting water activity within the formulation and
retarding interaction with the moisture sensitive API.
The inclusion of Starch 1500 in the formulation provides
exceptional stability for moisture sensitive APIs by reducing
or eliminating the detrimental effects of other excipients.
Impact of Excipients on Stability of Moisture Sensitive API (acetylesalycylic acid)
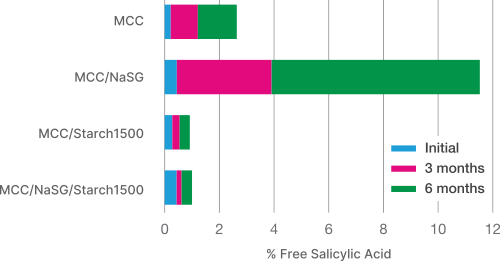
Low Water Activity
While Starch 1500 has a high moisture content, it has very low water activity, this gives better stability to moisture sensitive actives. Starch 1500 has bound moisture rather than free water available for reaction, as with other excipients.
Moisture scavenging properties of Starch 1500 makes it an excellent excipient to enhance stability of moisture sensitive drugs.
Comparisonof Water Activity vs Loss on Drying (LOD))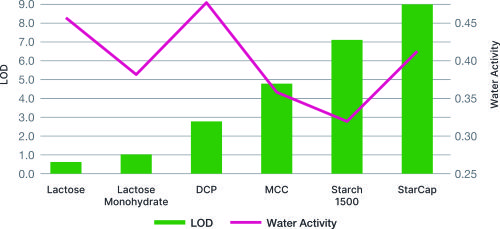
The use of Starch 1500 provides exceptional stability in moisture sensitive applications.
High Quality Tablets for Film Coating
Starch 1500 provides tablet hardness and low friability ideal for film coating and packaging.
Designed For Pharmaceutical Use
Manufactured in dedicated GMP facilities, Starch 1500 is a globally acceptable specialty excipient.
• Inert material - No interaction with reactive API
• Stabilizes moisture sensitive APIs
• Global regulatory acceptance